Ra-Hoor-Khuit Network's
Health
Schlick, G. and D.L. Bubenheim. 1996. Quinoa: Candidate crop for NASA's Controlled Ecological Life Support Systems. p. 632-640. In: J. Janick (ed.), Progress in new crops. ASHS Press, Arlington, VA.
Quinoa: Candidate Crop for NASA's Controlled Ecological Life Support Systems
Greg Schlick and David L. Bubenheim
- METHODOLOGY
- RESULTS AND DISCUSSION
- CONCLUSIONS
- REFERENCES
- Table 1
- Table 2
- Table 3
- Table 4
- Table 5
- Table 6
- Table 7
- Table 8
- Fig. 1
Quinoa (Chenopodium quinoa Willd.) is being considered as a potential "new" crop for NASA's Controlled Ecological Life Support System (CELSS). The CELSS concept will utilize plants to remove carbon dioxide from the atmosphere and generate food, oxygen, and water for the crew of long-term human space missions. Criteria for selection of potential crops include nutritional composition, harvest index, canopy stature, and life cycle duration.
Quinoa is a potential crop for CELSS due to it's high productivity and desirable nutritional characteristics. Of special interest is quinoa's high protein and mineral concentrations, in addition, the amino acid is suitable for a balanced human diet. Typically, CELSS has had to combine the nutritional values of several crops to obtain a suitable concentrations of amino acids for a human diet; quinoa may serve as a suitable complement to a balanced human diet.
Quinoa seeds have an exceptionally well balanced amino acid profile. For example, lysine, an essential amino acid deficient in most grain crops exceeds the concentrations necessary for proper amino acid nutrition in humans. In addition, the sulfur containing amino acids, cystine, and methionine are found in concentrations that are unusually high compared to other plants. Many minerals are found at concentrations greater than that reported for most grain crops; providing they are found in bioavailable forms, calcium, magnesium, and potassium are found in sufficient quantities for a balanced human diet. Most of the other minerals necessary for a balanced diet are found in greater concentrations than in most other crops as well. The pericarp of the quinoa seed contains saponins which may useful in the human diet and other applications for long-term space travel.
Conventionally, the seeds of quinoa are considered to be the edible form of the plant while the leaves have generally been overlooked. The leaves and sprouts can be eaten raw or cooked and provide a substantial amount of nutritive value. Utilizing quinoa as a leafy vegetable and a grain crop may provide nutritional and harvest versatility greater than that of a purely vegetative crop or that of a crop grown solely for it's seeds.
Significant increases in yield potential and nutrient content is attainable from crop plants grown in controlled environments (Bubenheim 1991). Ultimately, for a fully regenerative CELSS to be achievable we must maximize the productivity and quality of a each crop while minimizing the volume, mass, time and energy inputs. Because of the significant food value associated with quinoa the feasibility of controlled environment culture has been investigated. This manuscript summarizes efforts to select appropriate cultivars of quinoa for controlled environment production and efforts to determine optimal growing conditions.
METHODOLOGY
Cultivar Selection in the Greenhouse
Ten different accessions of quinoa were examined for potential use in controlled environment hydroponic production systems. The genotypes evaluated in the initial screening and their origins were: QL3 and QH3 from the Univ. of Cambridge, England; CO407XISLUGA and CO407 Heat Tolerant Population 1 from Colorado State University, Fort Collins, Colorado; 'Real', 'Sajama', and 'Cochabamba 250' from Bolivia; 'Blanca de Junin' from Peru; and 'Faro', and 'Temuco' from Chile. The primary selection criteria for advancement of the genotypes in the selection process were canopy height, biomass production, seed yield, harvest index, and life cycle duration.
Because of the general lack of experience anywhere in the hydroponic culture of quinoa, the preliminary trials focused on providing an appropriate nutritional regime. Following several seed-to-seed trials the candidate accession list was trimmed to four: 'Real', CO407, CO407 Heat Tolerant Population 1, and QL3. The other cultivars exhibited extreme growth habits under all conditions and were eliminated from the screening early in the process. A detailed characterization of the crop physiology of quinoa grown in controlled environment, hydroponic production was pursued using the four cultivars and results are presented only for those genotypes. A ranking was developed through a series of production studies while general responses to environmental factors such as photoperiod, nutrient composition, planting density, and temperature were determined. The top ranking cultivar was the first to be advanced to growth chamber trials.
Results of greenhouse studies, presented in Fig. 1 and Table 2, were all from plants grown in a greenhouse using a recirculating, deep-film hydroponic system maintained at 21°(± 3.0°C) with elevated carbon dioxide levels 1000 mmol mol-1 (Schlick and Bubenheim 1993). The plants were grown under both long and short day conditions (daily photon flux of 49.7 and 15.3 mol m-2 d-1, respectively). The nutrient solution (Table 1) was maintained at a pH of 5.8 and a electrical conductivity of 0.50 mS (seedling solution) during the first 20 days and then increased to an electrical conductivity of 1.50 mS for the duration of the experiment. The nutrient solution was delivered to the hydroponic trays at a flow rate of 14 liters min-1.
Growth Chamber Yield Studies
The initial efforts to define the yield potential for quinoa in highly controlled environments were focused on a series of experiments designed to determine photoperiod and irradiance responses. Three nearly identical growth chambers (EGC Model M-12) each with approximately 0.8 m2 of growing area were modified to increase the number of environmental variables controlled and to better maintain those conditions in the growing environment. The standard C-3 microprocessor (standard EGC control system) was removed and replaced with a Optomux analog and digital brain board (Opto 22) which supplied and received the input/output signal to and from a computer running Paragon 500 Control Software (Intec Controls Corp.). The control package allowed precise and complete computer control for all the environmental parameters.
The photoperiod response of quinoa was quantified by presenting three photoperiod treatments (16, 12, and 8 h) while maintaining the daily photosynthetic photon flux consistent among all photoperiods at 51.8 mol m-2 d-1. The instantaneous photosynthetic photon flux levels maintained in the chambers were 800, 1200, and 1800 µmol m-2 s-1, respectively. In a subsequent trial, designed to quantify the irradiance response of quinoa, photoperiod was maintained at 8 h with irradiance treatments of 800, 1200, and 1800 µmol m-2 s-1. The daily photosynthetic photon flux was 25.9, 34.6 and 51.8 mol m-2 d-1, respectively.
In all of the growth chamber studies, the relative humidity was maintained at 80% (±0.1% RH), temperature was maintained at 21°(±0.5°C), and the carbon dioxide level was maintained at 1000 mmol mol-1 (±10 mmol mol-1). The root zone environment consisted of a continually recirculating deep-film solution with a delivery rate of 14 liters min-1. The nutrient solution (Table 1) was maintained at a pH of 5.8 and the electrical conductivity was maintained at 0.50 mS during the first 20 days and then increased to 1.50 mS for the remainder of the experiment.
Assessing Growth and Yield
For both the greenhouse and growth chamber experiments growth, development, and morphology were characterized over time. Individual plants in the communities were measured weekly for height, branch number, internode length and leaf area index. At the same time, the days to floral initiation, days to flowering, duration of seed fill, and days to maturity were determined. At harvest, the plants were oven dried at 60°C to a uniform weight before dry mass was determined for seeds, leaves, stems, and roots, and yield analysis performed.
Analysis of Nutritional Value
Total proximate analysis, mineral composition, and amino acid profile were determined for each of the separated tissues: roots, leaves, stems, and seeds. Fatty acid profile, carbohydrate profile, total starch content, ascorbic acid (vitamin C), thiamin (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), vitamin A, a-tocopherol (vitamin E), and beta-carotene analysis was, at this time, only conducted on the seed and leaf tissue.
RESULTS AND DISCUSSION
Cultivar Determination
It is critical for a candidate CELSS crop to meet certain criteria which include small canopy stature, high harvest index and a favorable nutritional composition. While most of the cultivars examined exhibited a positive response to controlled environment production in reference to harvest index and nutritional composition, many grew exceedingly tall, some growing as tall as 2.5 m in height. Fig. 1 shows the height characteristics of four of the examined accessions. The size limitations of controlled environment chambers and eventually the size constraints of extra-terrestrial crop production systems force volume/height considerations in cultivar selection. Of the examined cultivars, 'Real' and QL3 showed the greatest promise in canopy stature, biomass partitioning, and seed yields (Table 2). 'Real' exhibited short height (60 cm) and maintained relative seed yield under short day conditions. The height of the QL3 canopy was similar at 58 cm under short days but was also the shortest under long day conditions. Additionally, QL3 exhibited little branching and consistently had the highest seed yield and harvest index under both long and short day conditions, compared with the other cultivars evaluated. The performance of QL3 in controlled environment production presented here is equivalent to or surpasses the performance of other crops selected as primary candidates for CELSS. The canopy height, yield, biomass accumulation rate, life cycle duration, and harvest index are all appropriate for continued consideration of quinoa as a CELSS crop and greater definition of yield potential is justified.
Planting Density
Previous crop production studies using controlled environment hydroponic systems (Bubenheim 1991) demonstrated that dramatic increases in yield and harvest index resulted in part from a higher planting densities when compared to average field seeding rates. In controlled environments, higher planting densities result in improved light interception and increased yields. Optimal field planting density reported for quinoa is approximately 32 plants m-2 (Johnson 1985). From our yield per unit area, harvest index, and biomass accumulation data, it is apparent that a planting density of approximately 140 plants m-2 appears to be optimal for hydroponic controlled environment production (data not shown).
Photoperiod and Irradiance Effects on Canopy Height, Biomass Accumulation and Allocation, and Yield
It appears that QL3 responds favorably to a combination of high irradiance (1800 µmol m-2 s-1) and short day lengths (8 h). Maintaining a daily photon flux of 51.8 mol d-1 and increasing the photoperiod results in an increase in canopy height. Maintaining the same photoperiod and altering the instantaneous flux results in a similar height response. This suggests that the optimal photo conditions for maintaining a short stature, high seed yield, and maximum harvest index for QL3 is short days with high instantaneous flux.
Biomass production and allocation was also affected by photoperiod and irradiance (Table 3 and 4). Long days (12 and 16 h) and low instantaneous photon flux levels promoted biomass production. However, the allocation of biomass differed greatly depending on the photoperiod and irradiance environment. While both shoot mass (including seeds) and root mass increased with increased biomass accumulation, the proportion of the shoot mass partitioned to the seed was greater in the short photoperiod and high irradiance environments. Plants that grew under a short photoperiod had a relatively constant shoot/root ratio. The plants that grew under long day conditions exhibited smaller ratios. The harvest index (seed only) in the 8 h/1800 µmol m-2 s-1 treatment was greater than that of the other treatments.
Most of the attention to this crop has been focused around the seed, but it is plausible that the leaves may provide an additional source of food and nutrition. If the edible leaves are added into the harvest index calculation, the harvest index can exceed 80%. Alternate harvesting techniques need to be examined, such as leaf harvest during the vegetative growth phase, to determine the effects, if any, this has on seed fill and yield.
Nutritional Composition
The protein concentrations (Table 5) found in the seed (20.9%) of quinoa grown in controlled environments is consistently greater than the average values obtained from field studies (16.5%). The increase in seed protein values is likely due to increased nutrient availability characteristic of recirculating hydroponic culture systems and the decreased stress of controlled environments. The values shown in Table 5 have been corrected to show zero moisture content. Due to the lack of a specific nitrogen:protein conversion ratio, a factor of 6.25 was used to calculate protein content.
Lipid concentrations in the seed and leaves are consistent with that reported for field data. For the seed, 64% of the lipids are in the form of polyunsaturates, 17% are in the form of mono-unsaturates and the remainder are saturated (19%).
The ash content in the seeds of controlled environment produced plants is consistent with that of field data (5.5% and 6.4%, respectively), yet the ash content in leaves of controlled environment plants is higher than that observed in field trials (24.7% and 19.1%, respectively).
Crude fiber concentrations found in seed is comparable to field results (2.8% and 3.3%, respectively) presented by Koziol (1992). The content of fiber found in the leaves of quinoa grown in controlled environments (8.4%) is considerably lower than any of the previously reported fiber concentrations (13.7%) from field studies. The reason for the low concentrations in the leaves of the hydroponically grown quinoa is unknown at this time, but may be related to the decreased stress in the controlled environment.
Carbohydrate concentration is greater in the controlled environment grown quinoa (78.5% seeds; 52.8% leaves) than in quinoa grown under field conditions (69.3% seeds; 35.0% leaves) (Koziol 1992). The quality of the seed carbohydrate is not affected by controlled environment production; approximately 47.5% of the carbohydrates is in the form of starch, similar to the field results.
Table 6 summarizes the amino acid composition of quinoa from multiple field trials (Risi and Galwey 1984) and of the controlled environment studies completed at NASA/Ames Research Center. Of primary interest to CELSS is the high lysine and sulfur containing amino acid (methionine and cystine) concentrations. Currently, CELSS has found it necessary to combine nutritional values of crops such as soybeans (Glycine max) and wheat (Triticum aestivum) to obtain a suitable amino acid pattern that meets the needs of humans during long-term space travel. Amino acid analysis from field trials indicate quinoa as a good source of lysine, methionine, cystine as well as all the other essential amino acids and meets or exceeds the recommendations for a proper amino acid nutrition. In hydroponic production, all amino acid concentrations have remained similar or increased in concentration with the exception of the sulfur-containing amino acids. The relative decrease in cystine and methionine may be due to a number of factors, such as a sub-optimal sulfur content within the nutrient solution or a response to controlled environment production (lack of stress) which may suppress the formation of the sulfur metabolites. Sulfur concentrations in the nutrient solution and specific stress response is currently being studied to better understand the factors influencing sulfur containing amino acid synthesis in quinoa.
Quinoa has a very high mineral value and may prove to be beneficial for long-term human space travel. The mineral concentrations reported for quinoa seem to vary dramatically. This may due to the soil type and mineral composition of the region and/or fertilizer application. In a controlled environment, the nutrient composition can easily be modified and maintained to meet the demands required for optimal plant growth or human nutritional requirements. It may also be possible to modify the nutrient solution in order to manipulate the mineral concentrations found in the tissue of quinoa. Table 7 provides a comparison of quinoa grown hydroponically to those of field grown plants reported by Koziol (1992) and Souci (1994). Table 8 compares the mineral concentrations of quinoa with other major grains and leafy vegetable crops. Quinoa is a good source of calcium, phosphorus, iron, sodium, and potassium. Modifications made to the nutrient solution have a direct effect on the mineral concentrations in the tissues. This may prove to be an excellent way to provide the proper mineral requirements for human nutrition to the crew of a space mission.
Quinoa appears to be a good source of vitamin E (10.04 mg/100 g), which is substantially higher than other grain crops and a adequate thiamin (0.68 mg/100 g), which is found in similar concentrations to other grain crops and niacin (1.53 mg/100 g), which is lower than reported from other grain crops, but still in biologically significant amounts. While not presented here, many of the vitamin concentrations from quinoa grown in controlled environments increased from the values presented from field grown studies. Quinoa may prove to a valuable source for some of the vitamins required for a human nutrition during space travel.
The pericarp of the quinoa seed contains saponins, a plant glycoside. Saponins are a structurally diverse group of naturally occurring compounds found mainly in plants (Price et al. 1987). These saponins impart a bitter taste and tend to foam in aqueous solutions. Koziol (1992) reports concentrations ranging from 0.01%-4.65% for saponins (dry weight) in quinoa. Until recently, saponins have been considered to be highly toxic. While, extremely toxic to cold-blooded animals, the oral toxicity to mammals is low. Saponins present in common foods, including quinoa, seem to free from significant oral toxicity (Oser 1966; Ishaaya et al. 1969; Phillips et al. 1979; Malinow 1982 and 1984; Oakenfull and Sidhu 1990) in humans. Saponins naturally present in foodstuffs are non-toxic and suggests they may even be beneficial in a human diet. Ruales (1992) showed that the digestibility of quinoa protein is comparable to that of other high quality food proteins and that the saponins do not exert any negative effect on the nutritive quality of the protein.
However, because saponins may impart a bitter taste, the separation of saponins from quinoa seed is easily accomplished by rinsing the seed in cold alkaline water or by mechanical abrasion. Saponins are useful in producing soaps, detergents, shampoos, cosmetics, and pharmaceuticals (Johnson and Ward 1993). The broad spectrum of useful products made with saponins may prove to be beneficial for long-term space habitation.
CONCLUSIONS
Quinoa is clearly a candidate for inclusion among the suite of crops to be grown as part of a Controlled Ecological Life Support System for long-term space exploration. Quinoa responded well to controlled environment production practices with large increases in seed production, maintenance of short canopy stature, and increased harvest index, as compared with the field. Quinoa productivity is similar to that of other current candidate species. In addition, the very desirable nutritional composition, leading to the original interest in quinoa, seems to be enhanced in controlled environment production. The protein values increase and the relative proportions of amino acids meet or exceed the requirements of humans, except for cysteine and methionine. The mineral concentration is exceptional and has the potential of being easily manipulated to meet the dietary needs for human nutrition. The high protein levels, unique amino acid pattern, vitamins, minerals and desirable growth and yield features make quinoa a likely candidate crop for CELSS applications.
REFERENCES
- Bubenheim, D. 1991. Plants for water recycling, oxygen regeneration, and food production. Waste Management Res. 9:435-443.
- Ishaaya, I., Y. Birk, A. Bondi, and Y. Tencer. 1969. Soyabean saponins IX. Studies of their effect on birds, mammals and cold blooded organisms. J. Sci. Food Agr. 20:433-436.
- Johnson, D.L. and R. Croissant. 1985. Quinoa production in Colorado. Service In Action no.112. Colorado State Univ., Cooperative Extension, Fort Collins.
- Johnson, D.L. and S. Ward. 1993. Quinoa. p. 222-227. In: J. Janick and J.E. Simon (eds.), New crops. Wiley, New York.
- Koziol, M.J. 1992. Chemical composition and nutritional evaluation of quinoa. J. Food Comp. Anal. 5:35-68.
- Malinow, M., W. McNulty, D. Houghton, S. Kessler, P. Stenzel, S. Goodnight, E. Bardana, J. Polatay, P. McLaughlin, and A. Livingston. 1982. Lack of toxicity of alfalfa saponins in monkeys. J. Med. Primatol. 11:106-118.
- Malinow, M., P. McLaughlin, E. Bardana, S. Craig. 1984. Elimination of toxicity from diets containing alfalfa seeds. Food Cosmet. Toxicol. 22:583-587.
- Oakenfull, D. and G. Sidhu. 1990. Could saponins be a useful treatment for hypercholesterolaemia? Eur. J. Clin. Nut. 44:79-88.
- Oser, B. 1966. An evaluation of Yucca mohavensis as a source of food grade saponin. Food. Cosmet. Toxicol. 4:57-61.
- Phillips, J. K., Butterworth, I. Gaunt, J. Evans, and P. Grasso. 1979. Long-term toxicity study of quillaja extract in mice. Food Cosmet. Toxicol. 17:23-27.
- Risi, J. and N.W. Galwey. 1984. The Chenopodium grains of the Andes: Inca crops for modern agriculture. Adv. Appl. Biol. 10:145-217.
- Ruales, J. and B.M. Nair. 1992. Nutritional quality of the protein in quinoa (Chenopodium quinoa Willd) seeds. Plant Foods Hum. Nutr. 42(1):1-12.
- Schlick, G. and D.L. Bubenheim. 1993. Quinoa: An emerging "new" crop with potential for CELSS. NASA Tech. Paper #3422.
- Scott, M.L. 1986. Nutrition of humans and selected animal species. Wiley, New York.
- Souci, S.W., W. Fachmnn, and H. Kraut. 1994. Food composition and nutrition tables. CRC Press, Boca Raton, FL.
Table 1. Hydroponic nutrient solution for quinoa in controlled environments.
| Nutrient source |
Seedling soln. concn. |
Standard soln. concn. |
| Ca(NO3)2•4H2O |
0.5 mm |
2 mm |
| KNO3 |
0.5 mm |
2 mm |
| KH2PO4 |
0.15 mm |
0.6 mm |
| MgSO4•7H2O |
0.13 mm |
0.5 mm |
| K2SO4 |
0.13 mm |
0.5 mm |
| FeNO3•9H2O |
2.5 µm |
10 µm |
| FeCl3+HEDTA |
11.3 µm |
45 µm |
| MnCl2•4H2O |
0.75 µm |
3 µm |
| ZnSO4•7H2O |
0.75 µm |
3 µm |
| CuSO4•5H2O |
0.05 µm |
0.18 µm |
| Na2Mo4•2H2O |
0.02 µm |
0.09 µm |
| H3BO3 |
0.5 µm |
2 µm |
Table 2. Growth and yield characteristics of four selected quinoa cultivars grown in hydroponic greenhouse conditions.
| Cultivar |
Days to flower |
Days to harvest |
Harvest |
Total biomass |
| CO407 x ISLUGA |
24 |
103 |
0.37 |
542 |
| CO407 heat tol. |
27 |
103 |
0.17 |
453 |
| Real |
30 |
89 |
0.28 |
184 |
| QL3 |
27 |
89 |
0.49 |
503 |
Table 3. Total biomass allocation in quinoa cv. QL3 as influenced by photoperiod and irradiance.
|
Treatment |
||||||
|
Photoperiod (h) |
Instantaneous flux |
Daily flux (mol m-2s-1) |
Total biomass |
Shoot mass |
Root mass |
Shoot/root ratio |
|
8 |
1800 |
51.8 |
922±49.1 |
851±27.4 |
71±3.8 |
11.9 |
|
12 |
1200 |
51.8 |
1450±81.0 |
1314±32.4 |
136±6.4 |
8.9 |
|
16 |
900 |
51.8 |
1593±93.2 |
1444±49.5 |
149±5.2 |
9.7 |
|
8 |
1800 |
51.8 |
1021±102.3 |
944±51.5 |
76±11.2 |
12.4 |
|
8 |
1200 |
34.6 |
1336±87.9 |
1241±47.9 |
95±6.2 |
13.1 |
|
8 |
900 |
25.9 |
1546±108.0 |
1435±57.6 |
112±6.6 |
12.8 |
Table 4. Yield characteristics of quinoa cv. QL3 as influenced by photoperiod and irradiance.
|
Treatment |
Harvest index |
|||||
|
Photoperiod (h) |
Instantaneous flux |
Daily flux |
Seed yield (g/m) |
Leaf yield (g/m) |
seed only |
seed& |
|
8 |
1800 |
51.8 |
507±11.2 |
250±12.2 |
0.55 |
0.82 |
|
12 |
1200 |
51.8 |
542±19.3 |
526±17.1 |
0.34 |
0.67 |
|
16 |
900 |
51.8 |
406±17.1 |
521±17.4 |
0.28 |
0.64 |
|
8 |
1800 |
51.8 |
592±17.7 |
128±21.0 |
0.58 |
0.71 |
|
8 |
1200 |
34.6 |
494±10.2 |
432±14.6 |
0.37 |
0.69 |
|
8 |
900 |
25.9 |
557±22.6 |
499±8.2 |
0.36 |
0.68 |
Table 5. Proximate analysis of quinoa cv. QL3 grown in controlled environment and comparison with quinoa grown in a field environment (corrected for zero moisture).
| Growing condition |
Component (%) |
||||
|
Organ |
Proteinz |
Fat |
Ash |
Carbohydrate |
Crude fiber |
| Hydroponically grown | |||||
|
Seeds |
20.9 |
5.5 |
4.9 |
78.5 |
2.8 |
|
Stems |
11.2 |
0.7 |
7.2 |
57.8 |
27.2 |
|
Roots |
13.3 |
0.4 |
15.0 |
74.8 |
26.3 |
|
Leaves |
18.7 |
1.0 |
24.7 |
52.8 |
8.4 |
| Field growny | |||||
|
Seeds |
16.6 |
6.4 |
3.9 |
69.3 |
3.3 |
|
Leaves |
21.9 |
3.8 |
19.1 |
35.0 |
13.7 |
z
Total Kjeldahl NitrogenyKoziol 1992
Table 6. Amino acid composition of quinoa compared with wheat, soybean, skimmed milk, and amino acid requirements of humans.
|
Content (g/100g protein) |
|||||||
| Amino acid |
Hydroponic quinoa |
Field quinoaz seeds |
Wheatz |
Soybeanz |
Skimmed milkz |
Amino acid requirementsy |
|
|
leaves |
seeds |
||||||
| Isoleucine |
3.2 |
3.9 |
5.2 |
3.8 |
4.9 |
6.3 |
4.0 |
| Leucine |
5.6 |
6.4 |
6.7 |
6.8 |
7.6 |
9.7 |
6.7 |
| Lysine |
3.5 |
5.9 |
6.2 |
2.9 |
6.4 |
7.7 |
5.0 |
| Phenylalanine |
3.9 |
4.1 |
3.8 |
4.5 |
4.9 |
4.9 |
3.2 |
| Arginine |
4.0 |
9.4 |
7.9 |
4.8 |
7.2 |
3.7 |
2.0 |
| Histidine |
1.2 |
3.0 |
2.7 |
2.2 |
2.5 |
2.6 |
1.7 |
| Alanine |
3.9 |
4.0 |
4.4 |
3.8 |
4.3 |
4.0 |
-.- |
| Aspartic acid |
8.0 |
9.0 |
8.1 |
5.3 |
12.0 |
8.3 |
-.- |
| Glutamic acid |
14.0 |
15.0 |
14.0 |
27.0 |
18.0 |
23.0 |
-.- |
| Glycine |
4.3 |
5.3 |
5.7 |
4.0 |
4.2 |
2.2 |
-.- |
| Proline |
3.6 |
3.5 |
4.0 |
10.0 |
5.5 |
11.0 |
-.- |
| Serine |
3.6 |
4.4 |
4.6 |
5.0 |
5.6 |
6.0 |
-.- |
| Tyrosine |
2.6 |
3.2 |
3.1 |
3.1 |
3.5 |
5.0 |
3.2 |
| Phenylalanine + Tyrosine |
6.5 |
7.3 |
6.9 |
7.6 |
8.4 |
9.9 |
6.4 |
| Cysteine |
0.6 |
1.0 |
1.4 |
2.3 |
1.5 |
0.9 |
1.3 |
| Methionine |
0.8 |
1.0 |
1.4 |
1.7 |
1.4 |
2.5 |
1.9 |
| Threonine |
3.5 |
3.5 |
4.1 |
3.1 |
4.2 |
4.6 |
3.4 |
| Tryptophan |
1.6 |
1.1 |
1.2 |
1.1 |
1.3 |
1.4 |
1.1 |
| Valine |
4.0 |
4.5 |
4.6 |
4.7 |
5.0 |
6.9 |
4.6 |
| Cysteine + Methionine |
1.4 |
2.0 |
2.8 |
4.0 |
2.9 |
3.4 |
3.2 |
z
Souci et al. 1994yScott 1986
Table 7. Tissue analysis of all plant parts of quinoa grown in hydroponic solution and seed analysis in field conditions.
| Treatment |
P |
Mg |
K |
Ca |
S |
Na |
Zn |
Cu |
Fe |
Mn |
| Hydroponic | ||||||||||
|
Roots |
0.85 |
0.84 |
3.89 |
1.67 |
-.- |
1232 |
80 |
14 |
2076 |
159 |
|
Stems |
0.18 |
0.11 |
3.26 |
0.99 |
-.- |
49 |
27 |
0 |
89 |
27 |
|
Leaves |
0.37 |
0.94 |
11.81 |
2.92 |
0.69 |
159 |
88 |
4 |
293 |
353 |
|
Seeds |
0.27 |
0.92 |
2.17 |
0.16 |
0.24 |
36 |
94 |
2 |
186 |
48 |
| Field | ||||||||||
|
Seedz |
0.33 |
0.28 |
0.80 |
0.08 |
-.- |
96 |
25 |
0 |
79 |
28 |
|
Leavesy |
0.04 |
0.08 |
0.38 |
0.15 |
-.- |
2890 |
5 |
1 |
9 |
-- |
z
Souci et al. 1994yKoziol 1992
Table 8. Comparative mineral nutrition values of quinoa seeds and leaves with other major seed and leafy vegetable crops.
|
Content (mg/100g) |
|||||
| Crop |
Calcium |
Phosphorus |
Iron |
Sodium |
Potassium |
| Quinoa seed |
160 |
270 |
19 |
4 |
2170 |
| Wheat |
38 |
341 |
3 |
8 |
381 |
| Soybean |
201 |
550 |
7 |
5 |
1800 |
| Quinoa leaves |
2920 |
370 |
29 |
16 |
1181 |
| Spinach |
126 |
55 |
4 |
65 |
633 |
| Cabbage |
35 |
30 |
1 |
4 |
266 |
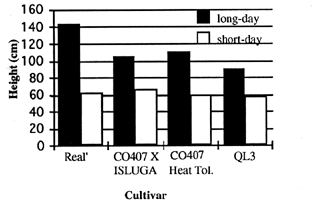
Fig. 1. Effect of daylength on the height characteristics of four selected C. quinoa cultivars grown in hydroponic greenhouse conditions.
Found on:
Quinoa Candidate Crop for NASA's Controlled Ecological Life Support Systems
This page last updated: 03/01/2018
